If you run pharma operations, you have seen how a “small” environmental shift can become a deviation, an investigation, or a rejected batch. Pharma humidity control is often the invisible variable behind those surprises, especially when standard solutions behave well in normal conditions but fail during real-world variability.
Here is the operational truth: a Pharma Manager wants zero batch rejection and GMP compliance, but faces unpredictable moisture risks with standard solutions. That is why the goal is not simply “lower humidity.” The goal is precision humidity control that acts like an insurance policy for product quality.
This guide covers why humidity matters, where risk hides, and how to build a practical humidity control system across pharmaceutical storage, processing, packaging, and distribution.

Why pharma humidity control is a quality variable, not a comfort setting
In pharma, humidity is not about comfort or facility preference. It is tied to product integrity and process consistency.
1) Moisture can change chemistry and performance quietly
Some drug substances and finished products are moisture-sensitive. Exposure can accelerate degradation pathways, shift dissolution behavior, or increase variability across lots. The most frustrating part is that early-stage signals are often subtle, then show up later in testing or stability.
2) Humidity drives physical failure modes that look like “process issues”
Even if potency remains acceptable, humidity can trigger physical failures that disrupt manufacturing:
-
powders caking or clumping
-
poor powder flow and inconsistent dispensing
-
tablet sticking, picking, or friability shifts
-
capsule shell softening or brittleness changes
-
static problems under low humidity that increase loss and handling variability
Teams often chase these as formulation or equipment problems, when the root cause is environmental drift and microenvironment exposure.
3) GMP compliance becomes harder to defend when humidity is unstable
If humidity is inconsistent, it becomes more difficult to justify environmental controls during audits, investigations, and deviation reviews. Stable controls reduce noise in your quality system and reduce the frequency of “unexplained” trends.
The biggest misconception: your product does not live in the room
Many teams treat humidity as one number on the wall. In reality there are two layers:
Layer A: Room RH (facility control)
This is the humidity your HVAC or dehumidification system targets. It matters, but it is only the baseline.
Layer B: Microenvironment RH (product control)
This is the humidity inside:
-
drums and liners
-
bins, totes, and intermediate containers
-
cartons, shippers, and secondary packaging
-
partially opened containers during staging and dispensing
Your product spends most of its time inside packaging systems, not in free room air. That is why pharma humidity control has to include microenvironments, especially when you care about pharmaceutical storage and long hold times.
Where moisture risk hides across the pharma workflow
1) Pharmaceutical storage: stable room, unstable containers
Warehouses and controlled rooms can still produce microenvironment drift because of:
-
frequent door cycles and airflow changes
-
staging in corridors or docks
-
partial-use opening and resealing
-
long dwell time in packaging with permeability
If you only manage room RH, you may still see moisture uptake inside containers during storage.
2) Active pharmaceutical ingredient storage: the clock starts after the first opening
Active pharmaceutical ingredient storage is especially sensitive when APIs or excipients are hygroscopic. Once opened, repeated exposure during scooping, weighing, and resealing becomes cumulative. Even short exposures can matter when repeated daily.
3) Dispensing and weighing: small exposures, repeated often
Dispensing rooms can be controlled, but the operational reality is frequent opening and handling. If you see inconsistent weights, flow issues, or clumping, humidity exposure during dispensing is a prime suspect.
4) Processing and hold steps: humidity interacts with time
Granulation, drying, coating, compression, encapsulation, and hold steps each have different vulnerability windows. A stable room RH does not always guarantee stable moisture content in the product during transitions.
5) Distribution: transitions create condensation risk
Moving between cold rooms, ambient docks, trucks, and receiving areas creates temperature swings. Those swings can cause condensation inside packaging or on container surfaces, which creates moisture shocks that are hard to predict without data.
How to control humidity in pharma: a practical three-layer system
The most reliable pharma humidity control strategies use three layers. This avoids over-investing in equipment while still protecting product microenvironments where it matters.
Layer 1: Define the humidity target based on product risk
Start with product and packaging needs:
-
What humidity range supports stability and performance?
-
Which steps are most sensitive to excursions?
-
Where do you see the most deviations, rejects, or rework?
Your humidity target should be tied to quality attributes and real workflow conditions, not simply a generic facility setting.
Layer 2: Control the room, but treat it as the baseline
HVAC and dehumidification reduce risk and improve consistency. But room control alone is not a guarantee for:
-
pharmaceutical storage in permeable packaging
-
long hold steps
-
partially opened APIs and intermediates
-
shipping and lane variability
Room RH is necessary. Microenvironment control is what makes it robust.
Layer 3: Protect the microenvironment with the right moisture tool
This is where teams choose between two fundamentally different approaches:
A) Traditional absorb-only solutions (common desiccants)
A classic pharmaceutical desiccant absorbs moisture and can be effective for many packaging scenarios. The limitation is that absorb-only systems can overshoot and keep drying, depending on volume, seal quality, temperature fluctuations, and load.
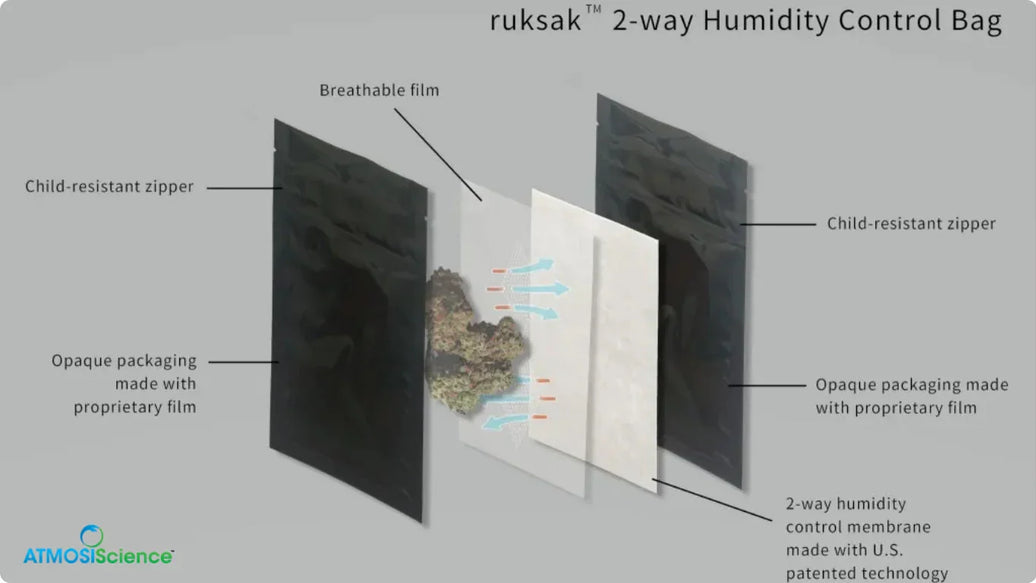
B) Setpoint-based buffering (two-way control)
Setpoint-based solutions are designed to buffer humidity up or down to maintain a defined RH window inside sealed packaging or containers. This matters when “drier” is not always better and when stability depends on staying within a controlled band.
In pharma, this distinction is important because some failure modes come from moisture gain, while others come from excessive dryness such as static issues or handling variability.
Selecting a pharmaceutical desiccant that works in real operations
When evaluating a pharmaceutical desiccant, do not stop at absorption capacity. In controlled environments, you need operational performance:
-
Control behavior
Does it stabilize humidity within a window, or does it continuously dry? -
Response speed
Can it correct excursions quickly after door openings or handling? -
Clean handling and safety
Low dusting, low residue, and minimal contamination risk are critical. -
Integration fit
Can it be used inside liners, drums, totes, cabinets, shippers, and secondary packaging without operational friction? -
Evidence and monitoring
Can you validate performance and trend excursions without repeatedly opening containers?
Practical playbook: implement humidity control without disrupting production
Step 1: Map moisture risk by product and workflow stage
Create a simple matrix:
-
product type (API, tablet, capsule, blister, vial)
-
packaging barrier quality
-
exposure points (staging time, dispensing, holds)
-
consequence severity (deviation risk, batch impact)
Step 2: Assign a control strategy by risk tier
-
Tier 1: Room control only
Low sensitivity products with strong packaging barriers. -
Tier 2: Room control plus microenvironment protection
Most use cases for pharmaceutical storage, intermediates, and partially opened containers. -
Tier 3: Room control plus microenvironment protection plus continuous monitoring
High sensitivity APIs, high-value products, and areas with recurring deviations.
Step 3: Apply microenvironment protection where it has the highest ROI
Common high-impact placements:
-
active pharmaceutical ingredient storage: headspace humidity protection inside drum liners
-
intermediate totes and bins: protection inside sealed tote or liner
-
secondary packaging: protection where permeability and dwell time create drift
-
shippers: protection for humidity variability across lanes and seasons
Step 4: Monitor smarter, not harder
Avoid “checking” by opening containers repeatedly. Instead:
-
use indicators where appropriate for quick checks
-
use loggers for excursion tracking and trending
-
trend by lane, season, and staging time to isolate patterns
Step 5: Validate like a quality team
Practical validation tests include:
-
recovery time after opening events
-
stability during a humidity spike and humidity drop scenario
-
documentation for replacement intervals, handling SOPs, and change control triggers
FAQ (keyword-optimized)
What is pharma humidity control?
Pharma humidity control is the set of facility and packaging measures used to keep humidity within a defined range so products remain stable, processes remain consistent, and GMP controls remain defensible.
Why is pharmaceutical storage humidity so difficult to manage?
Because pharmaceutical storage is not only about room RH. Microenvironments inside drums, liners, cartons, and shippers can drift due to permeability, long dwell times, partial-use opening, and temperature swings.
What is the best pharmaceutical desiccant for pharma applications?
The best pharmaceutical desiccant depends on your objective: absorb-only protection for moisture reduction, or setpoint-based buffering when you need to maintain a humidity window. Selection should be based on control behavior, speed, cleanliness, integration, and validation evidence.
Why does active pharmaceutical ingredient storage need special humidity controls?
Active pharmaceutical ingredient storage often involves hygroscopic materials and repeated opening events. Once a container is opened, cumulative exposure during staging, dispensing, and resealing increases the risk of moisture uptake and variability.
Closing: control humidity like you control risk
Humidity problems are expensive because they are rarely isolated. They multiply into deviations, investigations, rework, and shelf-life uncertainty.
The most resilient approach to pharma humidity control is simple in concept:
-
control the room as the baseline
-
protect the microenvironment where product actually lives
-
validate and monitor so the system stays defensible under GMP