ATMOSIScience proudly announces that the Fiber Humidity Pack, engineered and manufactured by Shanghai HengyuanMacromolecular Materials Co., Ltd., has achieved full certification under the rigorous U.S. Food and Drug Administration (FDA) regulation 21 CFR 176.170. This milestone strengthens ATMOSIScience’s position as a global leader in manufacturing safe, reliable, and environmentally sustainable humidity control materials for food, pharmaceutical, and consumer applications.

Comprehensive Overview of FDA Regulation 21 CFR 176.170
The FDA’s Code of Federal Regulations (CFR) Title 21, Part 176.170 specifically regulates the components of paper and paperboard that come into contact with food. The objective is to control extractable substances under typical contact conditions to ensure safety and prevent harmful migration into food products.
Paper-based materials can contain additives such as binders, coatings, sizing agents, and colorants necessary for their function. FDA 21 CFR 176.170 mandates that these components do not release harmful chemicals or exceed specific limits of extractable migrates during contact with aqueous (water-based), alcoholic, fatty, or acidic foods, which represent broad categories of potential consumer products.
Rigorous Testing & Validation of Fiber Humidity Pack
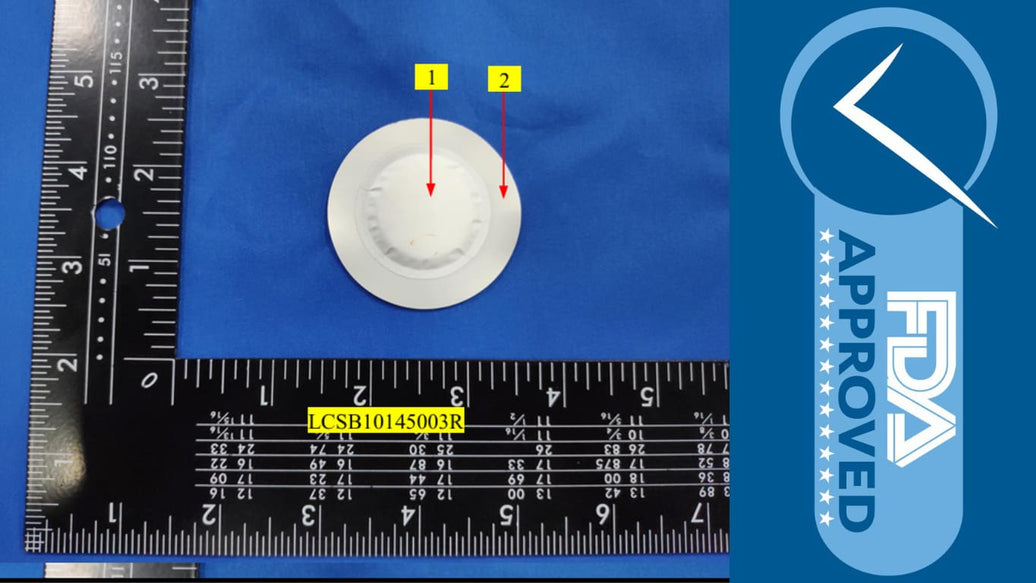
ATMOSIScience engaged Shenzhen Southern LCS Compliance Testing Co., Ltd., a renowned independent laboratory, to test the Fiber Humidity Pack extensively from October 13-20, 2025. The testing regime was designed to mirror worst-case prolonged exposure to food under elevated temperatures and varying solvent conditions to challenge the material’s chemical stability:
|
Test Parameter |
Solvent/Condition |
FDA Limit (mg/in²) |
Result (mg/in²) |
Safety Margin |
|
Water Extractives |
Water, 150°F, 2 hrs |
0.5 |
0.13 |
74% below limit |
|
n-Heptane Extractives |
n-Heptane, 100°F, 0.5 hrs |
0.5 |
0.13 |
74% below limit |
|
8% Alcohol Extractives |
8% Alcohol, 150°F, 2 hrs |
0.5 |
0.19 |
62% below limit |
|
50% Alcohol Extractives |
50% Alcohol, 150°F, 2 hrs |
0.5 |
0.21 |
58% below limit |
The consistent results show excellent chemical inertness with extractable substances well within regulatory limits, reassuring potential users on product safety.
Advanced Material Composition & Sustainability
The Fiber Humidity Pack is composed of premium cellulosic fibers sourced responsibly, combined with biodegradable binders that meet industry environmental standards. This product not only ensures compliance with FDA requirements but also aligns with FSSC 22000 food safety management standards, SGS RoHS environmental compliance, and industrial compostability under EN 13432 certification.
This triple validation ensures that while delivering high-performance moisture control, the product supports a circular economy with minimized environmental footprint—crucial for firms prioritizing sustainability.
Real-World Application Insights
Food Industry
Maintaining moisture balance in food packages extends product freshness, prevents mold growth, and delays spoilage, directly benefiting manufacturers, retailers, and consumers. The Fiber Humidity Pack addresses these industry needs with:
- Precise moisture buffering capacity
- Chemical safety assurance for direct food contact
- Compatibility with multiple packaging formats including vacuum and modified atmosphere packaging (MAP)
Pharmaceutical Packaging
Humidity control in pharmaceuticals is critical to preserve drug potency, stability, and shelf life. FDA certification guarantees that the Fiber Humidity Pack’s component materials pose no risk of chemical contamination, facilitating compliance with pharmaceutical packaging regulations.
Consumer Electronics & More
Humidity-sensitive electronics benefit from ATMOSIScience’s moisture-managed packaging solutions, which prevent corrosion and extend product lifespan without the use of hazardous substances.
Discover more our blog: Relative Humidity Control: Unlocking Efficiency Across Industries
Why ATMOSIScience Fiber Humidity Pack Stands Out
- FDA-Certified Safety: Verified compliance with 21 CFR 176.170 builds regulatory confidence and consumer trust.
- Environmental Certification: Supporting sustainability through compostable, RoHS, and food safety certifications.
- Unwavering Quality: Stringent quality control ensures consistent chemical stability, performance, and purity.
- Versatile Applications: Suitable for food, medical, electronic, and industrial moisture-sensitive packaging
Commitment to Quality and Future Innovation
ATMOSIScience continuously invests in research and development, leveraging advanced materials science, sustainability, and independent testing to maintain global leadership. The Fiber Humidity Pack exemplifies this dedication and empowers clients across industries to meet modern regulatory and environmental challenges efficiently.